Expeditors' Brussels Temperature Controlled Warehouse
Few products are more critical to deliver safely and on time than pharmaceuticals. Here, logistics can literally be the difference between life and death.
Pharmaceuticals is a high-stakes business. An ethical drugmaker starts with 25,000 possible substances, then sorts through them to find the one that can be manufactured at scale, compounded into a deliverable medicine, shipped to market, distributed to patients and, finally, cure the targeted disease.
Compliance and regulation
In addition to being big and fast, medical logistics need to be compliant. Documentation, security, handling, and storage must be just right.
So right, in fact, that many regulatory bodies require good manufacturing practice (GMP) certification from healthcare companies, who in turn require good distribution practice (GDP) certification from their logistics providers.
“Compliance and regulatory issues are huge,” says Dr. Theodore Stank at the University of Tennessee, citing one of a number of major challenges facing pharmaceutical companies when getting their product into a global marketplace.
“Britain’s decision to leave the EU, and the prospect of other countries following, could make this picture even more complex, especially if companies have to deal with a larger number of different regulatory and compliance regimes."
“Of course there are also extremely tight controls on the movement of pharmaceutical products. This regulates quality and prevents piracy, theft and bogus medicines entering the marketplace. Consignments must be registered, tracked, and temperature controlled among a series of compulsory stages.”
Stank says that businesses must develop systems to manage these areas of risk, while protecting the product in transit. Modern tracking systems working in real time have brought massive benefits to this process, he adds.
Tracking systems must continue to evolve, he says, as medicines become more specific to individuals and less “catch-all”. This will be particularly stark in a future where drugs are delivered directly to the patient’s door in an Amazon-style delivery service.
“Expeditors’ approach is to work to the highest standards in all locations, across the board, around the world,” explains Andy Faes, Regional Manager in our healthcare vertical. “Rather than sinking to the lowest common denominator, the company follows the strictest Good Distribution Practice (GDP) legislation by the European Commission, and then, specifically, those prescribed in the UK by the MHRA [Medicines and Healthcare products Regulatory Agency] and Ireland by the HPRA [Health Products Regulatory Authority] as being the highest level.”
Because Expeditors has grown organically and not by acquisition, customers and patients at the end of the logistics chain can be confident that the processes and controls applied in Singapore will be the same as those in San Diego, or Sao Paulo, or Shanghai – always identical.
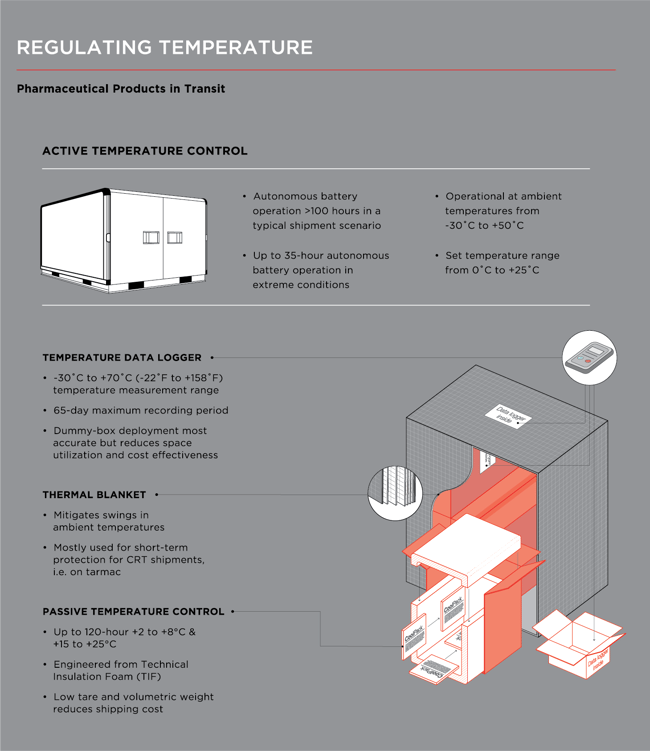
Faes identifies temperature control as one of the trickiest standards to uphold, relating a passive packaging solution that Expeditors implemented.
“For one customer in Germany, the company moves 600 daily shipments in passive packaging at +2°C to +8°C, around the EU and the world. Destinations include heat-intensive countries such as Saudi Arabia, so visibility and temperature monitors are key in ensuring the integrity of the product and GDP compliance. Our operational processes and quality program ensure we consistently meet or exceed the customers’ key metrics.”
Transatlantic Healthcare Service
Another proprietary offering in this sector is Expeditors’ Transatlantic Healthcare Service (THS). This is a dedicated daily air service connecting Europe with North America. The approach is to assess and manage risks via a door-to-door service.
Faes recounts how THS was invaluable in helping a producer of respiratory medicines move its entire weekly production of inhalers from France to the US state of Tennessee.
“In combination with Expeditors’ route-design tool, which maps out the timings and locations of product throughout the shipping process, the THS allowed a GDP-compliant transfer with minimum airport-to-airport transit time, secured daily capacity, and full visibility with temperature monitoring.
“The operation involved three fully pharma-loaded Boeing 777s flying from Brussels to Washington DC, and dedicated temperature-controlled trucks on the ground stretch. All went smoothly, with no dreaded ‘black holes’ occurring en route.” In both examples, GDP compliance was paramount to the customer and successfully achieved.
Please contact us if you would like to speak to one of our healthcare specialists.